The room 1 preschool children conducted an experiment called the “Disappearing eggshell”.
For this experiment, they used one brown egg, one white egg, and vinegar.
They made initial observations as they passed the eggs around, stating some of the differences: some noticed they have different colors, another noticed one is bigger than the other.
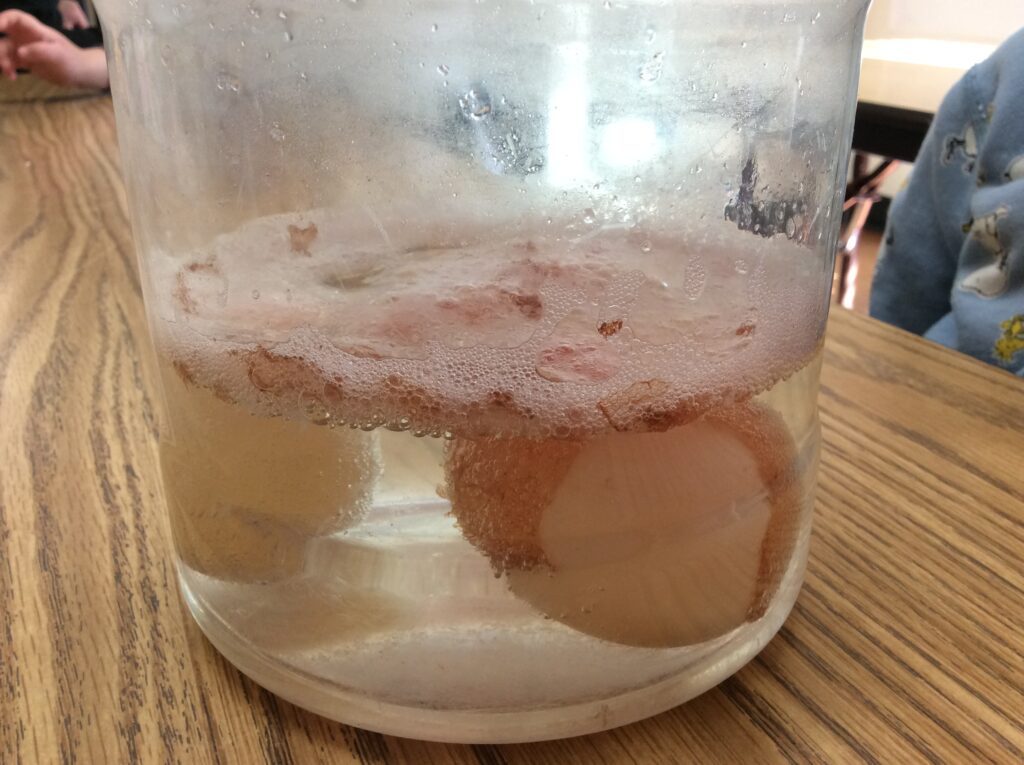
The teacher talked about the chemical reaction between the vinegar and the calcium carbonate in the eggshell, which causes the eggshell to dissolve. They poured vinegar over the eggs and observed bubbles forming around the shell!
Each child estimated a time frame for the eggshell to disappear, and also hypothesized if the color of the eggshell would affect how fast it dissolves in vinegar. Each child’s hypothesis was documented so they could refer to it later.
The children observed the eggs daily to monitor progress… First, they noticed a lot of bubbles and some brown substance floating on top of the vinegar. Then, they noticed that half of the white eggshell was dissolving and they could begin to see the egg yolk inside.
After 3 days, the eggshell had completely dissolved. The teacher washed the white egg and walked around to let others touch it. Some friends said that the egg was “squishy”!